Researchers working under DARPA’s Pandemic Preparedness Platform seek to create instant, short-term countermeasure
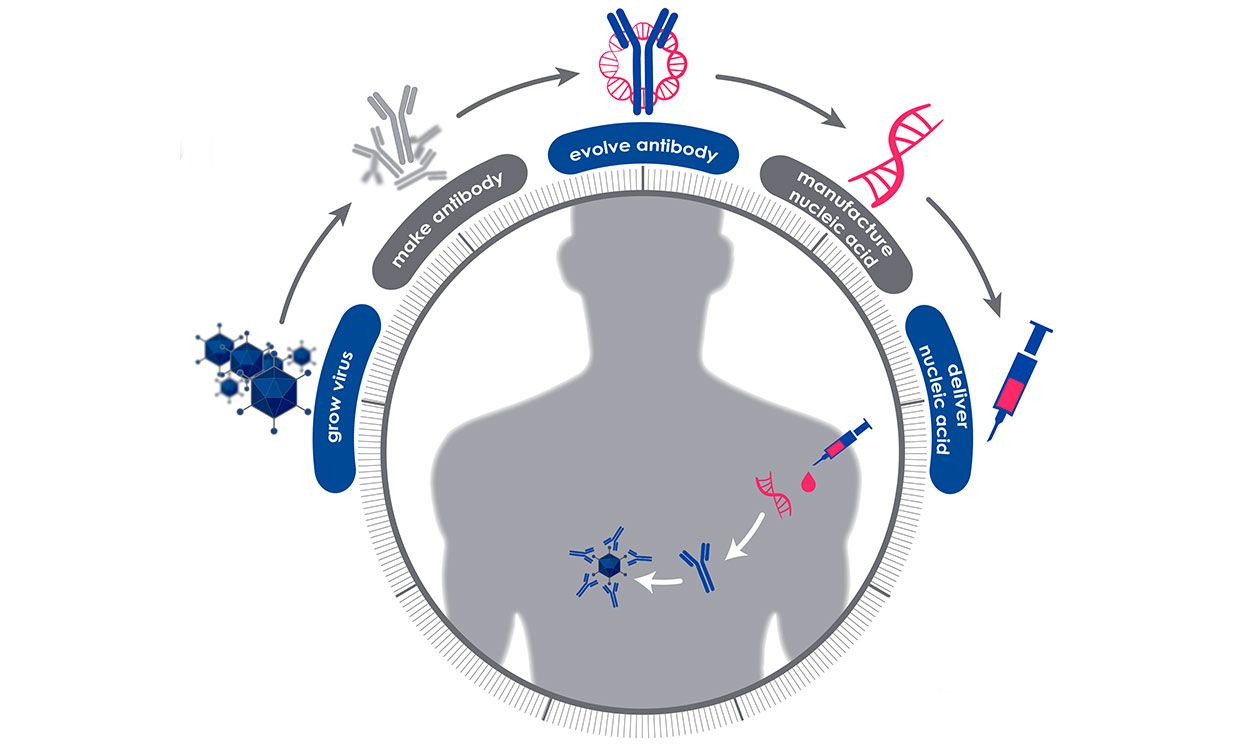
When DARPA launched its Pandemic Preparedness Platform (P3) program two years ago, the pandemic was theoretical. It seemed like a prudent idea to develop a quick response to emerging infectious diseases. Researchers working under the program sought ways to confer instant (but short-term) protection from a dangerous virus or bacteria.
Today, as the novel coronavirus causes a skyrocketing number of COVID-19 cases around the world, the researchers are racing to apply their experimental techniques to a true pandemic playing out in real time. “Right now, they have one shot on goal,” says DARPA program manager Amy Jenkins. “We’re really hoping it works.”
The P3 program’s plan was to start with a new pathogen and to “develop technology to deliver medical countermeasures in under 60 days—which was crazy, unheard of,” says Jenkins. The teams have proven they can meet this ambitious timeline in previous trials using the influenza and Zika viruses. Now they’re being asked to pull off the same feat with the new coronavirus, which more formally goes by the name SARS-CoV-2 and causes the illness known as COVID-19.
James Crowe, director of the Vanderbilt Vaccine Center at the Vanderbilt University Medical Center, leads one of the four P3 teams. He spoke with IEEE Spectrum from Italy, where he and his wife are currently traveling—and currently recovering from a serious illness. “We both got pretty sick,” Crowe says, “we’re pretty sure we already had the coronavirus.” For his wife, he says, it was the worst illness she’s had in three decades. They’re trying to get tested for the virus.
Ironically, Crowe is quite likely harboring the raw material that his team and others need to do their work. The DAPRA approach called for employing antibodies, the proteins that our bodies naturally use to fight infectious diseases, which remain in our bodies after an infection.
In the P3 program, the 60-day clock begins when a blood sample is taken from a person who has fully recovered from the disease of interest. Then the researchers screen that sample to find all the protective antibodies the person’s body has made to fight off the virus or bacteria. They use modeling and bioinformatics to choose the antibody that seems most effective at neutralizing the pathogen, and then determine the genetic sequence that codes for the creation of that particular antibody. That snippet of genetic code can then be manufactured quickly and at scale, and injected into people.
Jenkins says this approach is much faster than manufacturing the antibodies themselves. Once the genetic snippets are delivered by an injection, “your body becomes the bioreactor” that creates the antibodies, she says. The P3 program’s goal is to have protective levels of the antibodies circulating within 6 to 24 hours.
DARPA calls this a “firebreak” technology, because it can provide immediate immunity to medical personnel, first responders, and other vulnerable people. However, it wouldn’t create the permanent protection that vaccines provide. (Vaccines work by presenting the body with a safe form of the pathogen, thus giving the body a low-stakes opportunity to learn how to respond, which it remembers on future exposures.)
Robert Carnahan, who works with Crowe at the Vanderbilt Vaccine Center, explains that their method offers only temporary protection because the snippets of genetic code are messenger RNA, molecules that carry instructions for protein production. When the team’s specially designed mRNA is injected into the body, it’s taken up by cells (likely those in the liver) that churn out the needed antibodies. But eventually that RNA degrades, as do the antibodies that circulate through the blood stream.
“We haven’t taught the body how to make the antibody,” Carnahan says, so the protection isn’t permanent. To put it in terms of a folksy aphorism: “We haven’t taught the body to fish, we’ve just given it a fish.”
Carnahan says the team has been scrambling for weeks to build the tools that enable them to screen for the protective antibodies; since very little is known about this new coronavirus, “the toolkit is being built on the fly.” They were also waiting for a blood sample from a fully recovered U.S. patient. Now they have their first sample, and are hoping for more in the coming weeks, so the real work is beginning. “We are doggedly pursuing neutralizing antibodies,” he says. “Right now we have active pursuit going on.”
Jenkins says that all of the P3 groups (the others are Greg Semposki’s lab at Duke University, a small Vancouver company called AbCellera, and the big pharma company AstraZeneca) have made great strides in technologies that rapidly identify promising antibodies. In their earlier trials, the longer part of the process was manufacturing the mRNA and preparing for safety studies in animals. If the mRNA is intended for human use, the manufacturing and testing processes will be much slower because there will be many more regulatory hoops to jump through.
Crowe and Carnahan’s team has seen another project through to human trials; last year the lab worked with the biotech company Moderna to make mRNA that coded for antibodies that protected against the Chikungunya virus. “We showed it can be done,” Crowe says.
Moderna was involved in a related DARPA program known as ADEPT that has since ended. The company’s work on mRNA-based therapies has led it in another interesting direction—last week, the company made news with its announcement that it was testing an mRNA-based vaccine for the coronavirus. That vaccine works by delivering mRNA that instructs the body to make the “spike” protein that’s present on the surface of the coronavirus, thus provoking an immune response that the body will remember if it encounters the whole virus.
While Crowe’s team isn’t pursuing a vaccine, they are hedging their bets by considering both the manufacture of mRNA to code for the protective antibodies, and manufacturing the antibodies themselves. Crowe says that the latter process is typically slower (perhaps 18 to 24 months), but he’s talking with companies that are working on ways to speed it up. The direct injection of antibodies is a standard type of immunotherapy.
Crowe’s convalescence in Italy isn’t particularly relaxing. He’s working long days, trying to facilitate the shipment of samples to his lab from Asia, Europe, and the United States, and he’s talking to pharmaceutical companies that could manufacture whatever his lab comes up with. “We have to figure out a cooperative agreement for something we haven’t made yet,” he says, “but I expect that this week we’ll have agreements in place.”
Manufacturing agreements aren’t the end of the story, however. Even if everything goes perfectly for Crowe’s team and they have a potent antibody or mRNA ready for manufacture by the end of April, they’d have to get approval from the U.S. Food and Drug Administration. To get a therapy approved for human use typically takes years of studies on toxicity, stability, and efficacy. Crowe says that one possible shortcut is the FDA’s compassionate use program, which allows people to use unapproved drugs in certain life-threatening situations.
Gregory Poland, director of the Mayo Clinic Vaccine Research Group, says that mRNA-based prophylactics and vaccines are an important and interesting idea. But he notes that all talk of these possibilities should be considered “very forward-looking statements.” He has seen too many promising candidates fail after years of clinical trials to get excited about a new bright idea, he says.
Poland also says the compassionate use shortcut would likely only be relevant “if we’re facing a situation where something like Wuhan is happening in a major city in the U.S., and we have reason to believe that a new therapy would be efficacious and safe. Then that’s a possibility, but we’re not there yet,” he says. “We’d be looking for an unknown benefit and accepting an unknown risk.”
Yet the looming regulatory hurdles haven’t stopped the P3 researchers from sprinting. AbCellera, the Vancouver-based biotech company, tells IEEE Spectrum that its researchers have spent the last month looking at antibodies against the related SARS virus that emerged in 2002, and that they’re now beginning to study the coronavirus directly. “Our main discovery effort from a COVID-19 convalescent donor is about to be underway, and we will unleash our full discovery capabilities there,” a representative wrote in an email.
At Crowe’s lab, Carnahan notes that the present outbreak is the first one to benefit from new technologies that enable a rapid response. Carnahan points to the lab’s earlier work on the Zika virus: “In 78 days we went from a sample to a validated antibody that was highly protective. Will we be able to hit 78 days with the coronavirus? I don’t know, but that’s what we’re gunning for,” he says. “It’s possible in weeks and months, not years.”